Active Cells Must Continually Produce Atp
Identify the reactants and products of cellular respiration and where these reactions occur in a cell
Now that we've learned how autotrophs like plants convert sunlight to sugars, let's take a look at how all eukaryotes—which includes humans!—make use of those sugars.
In the process of photosynthesis, plants and other photosynthetic producers create glucose, which stores energy in its chemical bonds. Then, both plants and consumers, such as animals, undergo a series of metabolic pathways—collectively called cellular respiration. Cellular respiration extracts the energy from the bonds in glucose and converts it into a form that all living things can use.
Learning Objectives
- Describe the process of glycolysis and identify its reactants and products
- Describe the process of pyruvate oxidation and identify its reactants and products
- Describe the process of the citric acid cycle (Krebs cycle) and identify its reactants and products
- Describe the respiratory chain (electron transport chain) and its role in cellular respiration
Cellular respiration is a process that all living things use to convert glucose into energy. Autotrophs (like plants) produce glucose during photosynthesis. Heterotrophs (like humans) ingest other living things to obtain glucose. While the process can seem complex, this page takes you through the key elements of each part of cellular respiration.
Glycolysis
Glycolysis is the first step in the breakdown of glucose to extract energy for cellular metabolism. Nearly all living organisms carry out glycolysis as part of their metabolism. The process does not use oxygen and is therefore anaerobic (processes that use oxygen are called aerobic). Glycolysis takes place in the cytoplasm of both prokaryotic and eukaryotic cells. Glucose enters heterotrophic cells in two ways.
- Through secondary active transport in which the transport takes place against the glucose concentration gradient.
- Through a group of integral proteins called GLUT proteins, also known as glucose transporter proteins. These transporters assist in the facilitated diffusion of glucose.
Glycolysis begins with the six carbon ring-shaped structure of a single glucose molecule and ends with two molecules of a three-carbon sugar calledpyruvate(Figure 1).

Figure 1. Reactants and products of glycolysis.
Glycolysis consists of ten steps divided into two distinct halves. The first half of the glycolysis is also known as the energy-requiring steps. This pathway traps the glucose molecule in the cell and uses energy to modify it so that the six-carbon sugar molecule can be split evenly into the two three-carbon molecules. The second half of glycolysis (also known as the energy-releasing steps) extracts energy from the molecules and stores it in the form of ATP and NADH, the reduced form of NAD.
First Half of Glycolysis (Energy-Requiring Steps)

Figure 2. The first half of glycolysis uses two ATP molecules in the phosphorylation of glucose, which is then split into two three-carbon molecules.
Step 1. The first step in glycolysis is catalyzed by hexokinase, an enzyme with broad specificity that catalyzes the phosphorylation of six-carbon sugars. Hexokinase phosphorylates glucose using ATP as the source of the phosphate, producing glucose-6-phosphate, a more reactive form of glucose. This reaction prevents the phosphorylated glucose molecule from continuing to interact with the GLUT proteins, and it can no longer leave the cell because the negatively charged phosphate will not allow it to cross the hydrophobic interior of the plasma membrane.
Step 2. In the second step of glycolysis, an isomerase converts glucose-6-phosphate into one of its isomers, fructose-6-phosphate. Anisomerase is an enzyme that catalyzes the conversion of a molecule into one of its isomers. This change from phosphoglucose to phosphofructose allows the eventual split of the sugar into two three-carbon molecules.
Step 3. The third step is the phosphorylation of fructose-6-phosphate, catalyzed by the enzyme phosphofructokinase. A second ATP molecule donates a high-energy phosphate to fructose-6-phosphate, producing fructose-1,6-bisphosphate. In this pathway, phosphofructokinase is a rate-limiting enzyme. It is active when the concentration of ADP is high; it is less active when ADP levels are low and the concentration of ATP is high. Thus, if there is "sufficient" ATP in the system, the pathway slows down. This is a type of end product inhibition, since ATP is the end product of glucose catabolism.
Step 4. The newly added high-energy phosphates further destabilize fructose-1,6-bisphosphate. The fourth step in glycolysis employs an enzyme, aldolase, to cleave 1,6-bisphosphate into two three-carbon isomers: dihydroxyacetone-phosphate and glyceraldehyde-3-phosphate.
Step 5. In the fifth step, an isomerase transforms the dihydroxyacetone-phosphate into its isomer, glyceraldehyde-3-phosphate. Thus, the pathway will continue with two molecules of a single isomer. At this point in the pathway, there is a net investment of energy from two ATP molecules in the breakdown of one glucose molecule.
Second Half of Glycolysis (Energy-Releasing Steps)
So far, glycolysis has cost the cell two ATP molecules and produced two small, three-carbon sugar molecules. Both of these molecules will proceed through the second half of the pathway, and sufficient energy will be extracted to pay back the two ATP molecules used as an initial investment and produce a profit for the cell of two additional ATP molecules and two even higher-energy NADH molecules.

Figure 3. The second half of glycolysis involves phosphorylation without ATP investment (step 6) and produces two NADH and four ATP molecules per glucose.
Step 6. The sixth step in glycolysis (Figure 3) oxidizes the sugar (glyceraldehyde-3-phosphate), extracting high-energy electrons, which are picked up by the electron carrier NAD+, producing NADH. The sugar is then phosphorylated by the addition of a second phosphate group, producing 1,3-bisphosphoglycerate. Note that the second phosphate group does not require another ATP molecule.
Here again is a potential limiting factor for this pathway. The continuation of the reaction depends upon the availability of the oxidized form of the electron carrier, NAD+. Thus, NADH must be continuously oxidized back into NAD+ in order to keep this step going. If NAD+ is not available, the second half of glycolysis slows down or stops. If oxygen is available in the system, the NADH will be oxidized readily, though indirectly, and the high-energy electrons from the hydrogen released in this process will be used to produce ATP. In an environment without oxygen, an alternate pathway (fermentation) can provide the oxidation of NADH to NAD+.
Step 7. In the seventh step, catalyzed by phosphoglycerate kinase (an enzyme named for the reverse reaction), 1,3-bisphosphoglycerate donates a high-energy phosphate to ADP, forming one molecule of ATP. (This is an example of substrate-level phosphorylation.) A carbonyl group on the 1,3-bisphosphoglycerate is oxidized to a carboxyl group, and 3-phosphoglycerate is formed.
Step 8. In the eighth step, the remaining phosphate group in 3-phosphoglycerate moves from the third carbon to the second carbon, producing 2-phosphoglycerate (an isomer of 3-phosphoglycerate). The enzyme catalyzing this step is a mutase (a type of isomerase).
Step 9. Enolase catalyzes the ninth step. This enzyme causes 2-phosphoglycerate to lose water from its structure; this is a dehydration reaction, resulting in the formation of a double bond that increases the potential energy in the remaining phosphate bond and produces phosphoenolpyruvate (PEP).
Step 10. The last step in glycolysis is catalyzed by the enzyme pyruvate kinase (the enzyme in this case is named for the reverse reaction of pyruvate's conversion into PEP) and results in the production of a second ATP molecule by substrate-level phosphorylation and the compound pyruvic acid (or its salt form, pyruvate). Many enzymes in enzymatic pathways are named for the reverse reactions, since the enzyme can catalyze both forward and reverse reactions.
Outcomes of Glycolysis
Glycolysis starts with glucose and ends with two pyruvate molecules, a total of four ATP molecules and two molecules of NADH. Two ATP molecules were used in the first half of the pathway to prepare the six-carbon ring for cleavage, so the cell has a net gain of two ATP molecules and two NADH molecules for its use.
If the cell cannot catabolize the pyruvate molecules further, it will harvest only two ATP molecules from one molecule of glucose. Mature mammalian red blood cells are not capable ofaerobic respiration—the process in which organisms convert energy in the presence of oxygen—and glycolysis is their sole source of ATP. If glycolysis is interrupted, these cells lose their ability to maintain their sodium-potassium pumps, and eventually, they die.
The last step in glycolysis will not occur if pyruvate kinase, the enzyme that catalyzes the formation of pyruvate, is not available in sufficient quantities. In this situation, the entire glycolysis pathway will proceed, but only two ATP molecules will be made in the second half. Thus, pyruvate kinase is a rate-limiting enzyme for glycolysis.
In Summary: Glycolysis
Glycolysis is the first pathway used in the breakdown of glucose to extract energy. It was probably one of the earliest metabolic pathways to evolve and is used by nearly all of the organisms on earth. Glycolysis consists of two parts: The first part prepares the six-carbon ring of glucose for cleavage into two three-carbon sugars. ATP is invested in the process during this half to energize the separation. The second half of glycolysis extracts ATP and high-energy electrons from hydrogen atoms and attaches them to NAD+. Two ATP molecules are invested in the first half and four ATP molecules are formed by substrate phosphorylation during the second half. This produces a net gain of two ATP and two NADH molecules for the cell.
Figure 4 shows the entire process of glycolysis in one image:
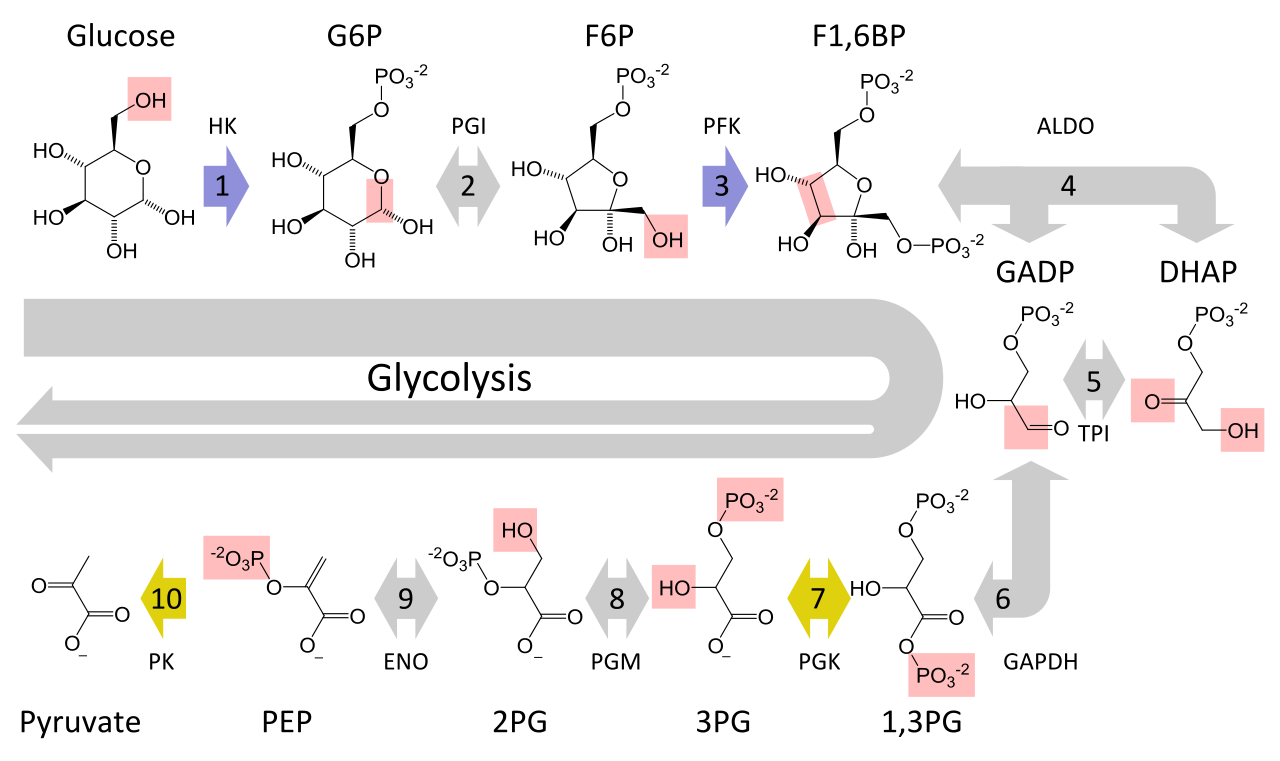
Figure 4. Glycolysis
Pyruvate Oxidation
If oxygen is available, aerobic respiration will go forward. In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria, which are the sites of cellular respiration. There, pyruvate will be transformed into an acetyl group that will be picked up and activated by a carrier compound called coenzyme A (CoA). The resulting compound is called acetyl CoA. CoA is made from vitamin B5, pantothenic acid. Acetyl CoA can be used in a variety of ways by the cell, but its major function is to deliver the acetyl group derived from pyruvate to the next stage of the pathway in glucose catabolism.
Breakdown of Pyruvate
In order for pyruvate (which is the product of glycolysis) to enter the Citric Acid Cycle (the next pathway in cellular respiration), it must undergo several changes. The conversion is a three-step process (Figure 5).

Figure 5. Upon entering the mitochondrial matrix, a multi-enzyme complex converts pyruvate into acetyl CoA. In the process, carbon dioxide is released and one molecule of NADH is formed.
Step 1. A carboxyl group is removed from pyruvate, releasing a molecule of carbon dioxide into the surrounding medium. The result of this step is a two-carbon hydroxyethyl group bound to the enzyme (pyruvate dehydrogenase). This is the first of the six carbons from the original glucose molecule to be removed. This step proceeds twice (remember: there are two pyruvate molecules produced at the end of glycolysis) for every molecule of glucose metabolized; thus, two of the six carbons will have been removed at the end of both steps.
Step 2. NAD+ is reduced to NADH. The hydroxyethyl group is oxidized to an acetyl group, and the electrons are picked up by NAD+, forming NADH. The high-energy electrons from NADH will be used later to generate ATP.
Step 3. An acetyl group is transferred to conenzyme A, resulting in acetyl CoA. The enzyme-bound acetyl group is transferred to CoA, producing a molecule of acetyl CoA.
Note that during the second stage of glucose metabolism, whenever a carbon atom is removed, it is bound to two oxygen atoms, producing carbon dioxide, one of the major end products of cellular respiration.
Acetyl CoA to CO2
In the presence of oxygen, acetyl CoA delivers its acetyl group to a four-carbon molecule, oxaloacetate, to form citrate, a six-carbon molecule with three carboxyl groups; this pathway will harvest the remainder of the extractable energy from what began as a glucose molecule. This single pathway is called by different names, but we will primarily call it the Citric Acid Cycle.
In Summary: Pyruvate Oxidation
In the presence of oxygen, pyruvate is transformed into an acetyl group attached to a carrier molecule of coenzyme A. The resulting acetyl CoA can enter several pathways, but most often, the acetyl group is delivered to the citric acid cycle for further catabolism. During the conversion of pyruvate into the acetyl group, a molecule of carbon dioxide and two high-energy electrons are removed. The carbon dioxide accounts for two (conversion of two pyruvate molecules) of the six carbons of the original glucose molecule. The electrons are picked up by NAD+, and the NADH carries the electrons to a later pathway for ATP production. At this point, the glucose molecule that originally entered cellular respiration has been completely oxidized. Chemical potential energy stored within the glucose molecule has been transferred to electron carriers or has been used to synthesize a few ATPs.
Citric Acid Cycle
Like the conversion of pyruvate to acetyl CoA, the citric acid cycle takes place in the matrix of mitochondria.This single pathway is called by different names: the citric acid cycle (for the first intermediate formed—citric acid, or citrate—when acetate joins to the oxaloacetate), the TCA cycle (since citric acid or citrate and isocitrate are tricarboxylic acids), and the Krebs cycle, after Hans Krebs, who first identified the steps in the pathway in the 1930s in pigeon flight muscles.
Almost all of the enzymes of the citric acid cycle are soluble, with the single exception of the enzyme succinate dehydrogenase, which is embedded in the inner membrane of the mitochondrion. Unlike glycolysis, the citric acid cycle is a closed loop: The last part of the pathway regenerates the compound used in the first step. The eight steps of the cycle are a series of redox, dehydration, hydration, and decarboxylation reactions that produce two carbon dioxide molecules, one GTP/ATP, and reduced forms of NADH and FADH2 (Figure 6). This is considered an aerobic pathway because the NADH and FADH2 produced must transfer their electrons to the next pathway in the system, which will use oxygen. If this transfer does not occur, the oxidation steps of the citric acid cycle also do not occur. Note that the citric acid cycle produces very little ATP directly and does not directly consume oxygen.

Figure 6. In the citric acid cycle, the acetyl group from acetyl CoA is attached to a four-carbon oxaloacetate molecule to form a six-carbon citrate molecule. Through a series of steps, citrate is oxidized, releasing two carbon dioxide molecules for each acetyl group fed into the cycle. In the process, three NAD+ molecules are reduced to NADH, one FAD molecule is reduced to FADH2, and one ATP or GTP (depending on the cell type) is produced (by substrate-level phosphorylation). Because the final product of the citric acid cycle is also the first reactant, the cycle runs continuously in the presence of sufficient reactants. (credit: modification of work by "Yikrazuul"/Wikimedia Commons)
Steps in the Citric Acid Cycle
Step 1. Prior to the start of the first step, pyruvate oxidation must occur. Then, the first step of the cycle begins: This is a condensation step, combining the two-carbon acetyl group with a four-carbon oxaloacetate molecule to form a six-carbon molecule of citrate. CoA is bound to a sulfhydryl group (-SH) and diffuses away to eventually combine with another acetyl group. This step is irreversible because it is highly exergonic. The rate of this reaction is controlled by negative feedback and the amount of ATP available. If ATP levels increase, the rate of this reaction decreases. If ATP is in short supply, the rate increases.
Step 2. In step two, citrate loses one water molecule and gains another as citrate is converted into its isomer, isocitrate.
Step 3. In step three, isocitrate is oxidized, producing a five-carbon molecule, α-ketoglutarate, together with a molecule of CO2 and two electrons, which reduce NAD+ to NADH. This step is also regulated by negative feedback from ATP and NADH, and a positive effect of ADP.
Steps 3 and 4. Steps three and four are both oxidation and decarboxylation steps, which release electrons that reduce NAD+ to NADH and release carboxyl groups that form CO2 molecules. α-Ketoglutarate is the product of step three, and a succinyl group is the product of step four. CoA binds the succinyl group to form succinyl CoA. The enzyme that catalyzes step four is regulated by feedback inhibition of ATP, succinyl CoA, and NADH.
Step 5. In step five, a phosphate group is substituted for coenzyme A, and a high-energy bond is formed. This energy is used in substrate-level phosphorylation (during the conversion of the succinyl group to succinate) to form either guanine triphosphate (GTP) or ATP. There are two forms of the enzyme, called isoenzymes, for this step, depending upon the type of animal tissue in which they are found. One form is found in tissues that use large amounts of ATP, such as heart and skeletal muscle. This form produces ATP. The second form of the enzyme is found in tissues that have a high number of anabolic pathways, such as liver. This form produces GTP. GTP is energetically equivalent to ATP; however, its use is more restricted. In particular, protein synthesis primarily uses GTP.
Step 6. Step six is a dehydration process that converts succinate into fumarate. Two hydrogen atoms are transferred to FAD, producing FADH2. The energy contained in the electrons of these atoms is insufficient to reduce NAD+ but adequate to reduce FAD. Unlike NADH, this carrier remains attached to the enzyme and transfers the electrons to the electron transport chain directly. This process is made possible by the localization of the enzyme catalyzing this step inside the inner membrane of the mitochondrion.
Step 7. Water is added to fumarate during step seven, and malate is produced. The last step in the citric acid cycle regenerates oxaloacetate by oxidizing malate. Another molecule of NADH is produced in the process.
Products of the Citric Acid Cycle
Two carbon atoms come into the citric acid cycle from each acetyl group, representing four out of the six carbons of one glucose molecule. Two carbon dioxide molecules are released on each turn of the cycle; however, these do not necessarily contain the most recently added carbon atoms. The two acetyl carbon atoms will eventually be released on later turns of the cycle; thus, all six carbon atoms from the original glucose molecule are eventually incorporated into carbon dioxide. Each turn of the cycle forms three NADH molecules and one FADH2 molecule. These carriers will connect with the last portion of aerobic respiration to produce ATP molecules. One GTP or ATP is also made in each cycle. Several of the intermediate compounds in the citric acid cycle can be used in synthesizing non-essential amino acids; therefore, the cycle is amphibolic (both catabolic and anabolic).
In Summary: Citric Acid Cycle
The citric acid cycle is a series of redox and decarboxylation reactions that remove high-energy electrons and carbon dioxide. The electrons temporarily stored in molecules of NADH and FADH2 are used to generate ATP in a subsequent pathway. One molecule of either GTP or ATP is produced by substrate-level phosphorylation on each turn of the cycle. There is no comparison of the cyclic pathway with a linear one.
Electron Transport Chain
You have just read about two pathways in cellular respiration—glycolysis and the citric acid cycle—that generate ATP. However, most of the ATP generated during the aerobic catabolism of glucose is not generated directly from these pathways. Rather, it is derived from a process that begins with moving electrons through a series of electron transporters that undergo redox reactions: the electron transport chain. This causes hydrogen ions to accumulate within the matrix space. Therefore, a concentration gradient forms in which hydrogen ions diffuse out of the matrix space by passing through ATP synthase. The current of hydrogen ions powers the catalytic action of ATP synthase, which phosphorylates ADP, producing ATP.
Electron Transport Chain
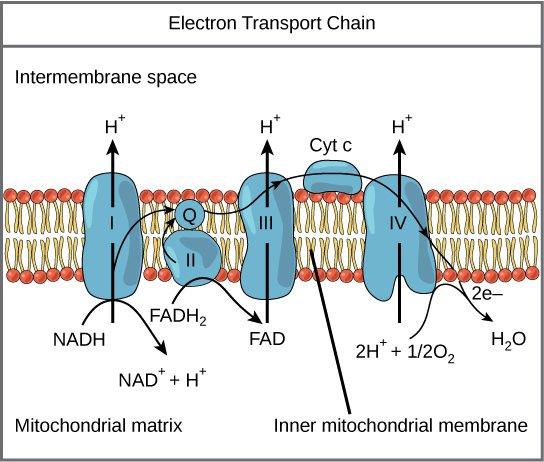
Figure 7. The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water.
The electron transport chain (Figure 7) is the last component of aerobic respiration and is the only part of glucose metabolism that uses atmospheric oxygen. Oxygen continuously diffuses into plants; in animals, it enters the body through the respiratory system. Electron transport is a series of redox reactions that resemble a relay race or bucket brigade in that electrons are passed rapidly from one component to the next, to the endpoint of the chain where the electrons reduce molecular oxygen, producing water. There are four complexes composed of proteins, labeled I through IV in Figure 7, and the aggregation of these four complexes, together with associated mobile, accessory electron carriers, is called the electron transport chain. The electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes. Note, however, that the electron transport chain of prokaryotes may not require oxygen as some live in anaerobic conditions. The common feature of all electron transport chains is the presence of a proton pump to create a proton gradient across a membrane.
Complex I
To start, two electrons are carried to the first complex aboard NADH. This complex, labeled I, is composed of flavin mononucleotide (FMN) and an iron-sulfur (Fe-S)-containing protein. FMN, which is derived from vitamin B2, also called riboflavin, is one of several prosthetic groups or co-factors in the electron transport chain. Aprosthetic group is a non-protein molecule required for the activity of a protein. Prosthetic groups are organic or inorganic, non-peptide molecules bound to a protein that facilitate its function; prosthetic groups include co-enzymes, which are the prosthetic groups of enzymes. The enzyme in complex I is NADH dehydrogenase and is a very large protein, containing 45 amino acid chains. Complex I can pump four hydrogen ions across the membrane from the matrix into the intermembrane space, and it is in this way that the hydrogen ion gradient is established and maintained between the two compartments separated by the inner mitochondrial membrane.
Q and Complex II
Complex II directly receives FADH2, which does not pass through complex I. The compound connecting the first and second complexes to the third isubiquinone (Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. Once it is reduced, (QH2), ubiquinone delivers its electrons to the next complex in the electron transport chain. Q receives the electrons derived from NADH from complex I and the electrons derived from FADH2 from complex II, including succinate dehydrogenase. This enzyme and FADH2 form a small complex that delivers electrons directly to the electron transport chain, bypassing the first complex. Since these electrons bypass and thus do not energize the proton pump in the first complex, fewer ATP molecules are made from the FADH2 electrons. The number of ATP molecules ultimately obtained is directly proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex III
The third complex is composed of cytochrome b, another Fe-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; this complex is also called cytochrome oxidoreductase. Cytochrome proteins have a prosthetic group of heme. The heme molecule is similar to the heme in hemoglobin, but it carries electrons, not oxygen. As a result, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe+ + (reduced) and Fe+ + + (oxidized). The heme molecules in the cytochromes have slightly different characteristics due to the effects of the different proteins binding them, giving slightly different characteristics to each complex. Complex III pumps protons through the membrane and passes its electrons to cytochrome c for transport to the fourth complex of proteins and enzymes (cytochrome c is the acceptor of electrons from Q; however, whereas Q carries pairs of electrons, cytochrome c can accept only one at a time).
Complex IV
The fourth complex is composed of cytochrome proteins c, a, and a3. This complex contains two heme groups (one in each of the two cytochromes, a, and a3) and three copper ions (a pair of CuA and one CuB in cytochrome a3). The cytochromes hold an oxygen molecule very tightly between the iron and copper ions until the oxygen is completely reduced. The reduced oxygen then picks up two hydrogen ions from the surrounding medium to make water (H2O). The removal of the hydrogen ions from the system contributes to the ion gradient used in the process of chemiosmosis.
Chemiosmosis
In chemiosmosis, the free energy from the series of redox reactions just described is used to pump hydrogen ions (protons) across the membrane. The uneven distribution of H+ ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient), owing to the hydrogen ions' positive charge and their aggregation on one side of the membrane.
If the membrane were open to diffusion by the hydrogen ions, the ions would tend to diffuse back across into the matrix, driven by their electrochemical gradient. Recall that many ions cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, hydrogen ions in the matrix space can only pass through the inner mitochondrial membrane through an integral membrane protein called ATP synthase (Figure 8). This complex protein acts as a tiny generator, turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient. The turning of parts of this molecular machine facilitates the addition of a phosphate to ADP, forming ATP, using the potential energy of the hydrogen ion gradient.
Practice Question
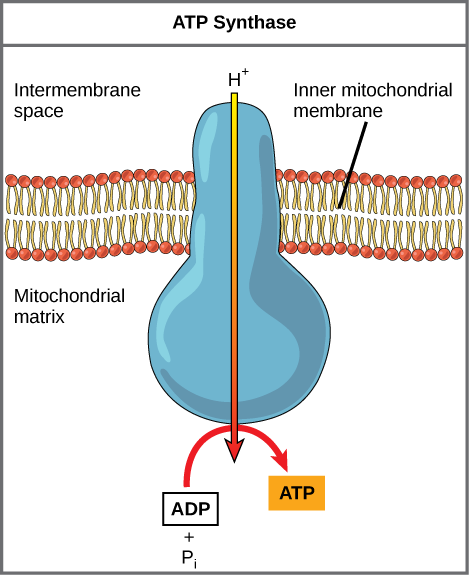
Figure 8. ATP synthase is a complex, molecular machine that uses a proton (H+) gradient to form ATP from ADP and inorganic phosphate (Pi). (Credit: modification of work by Klaus Hoffmeier)
Dinitrophenol (DNP) is an uncoupler that makes the inner mitochondrial membrane leaky to protons. It was used until 1938 as a weight-loss drug. What effect would you expect DNP to have on the change in pH across the inner mitochondrial membrane? Why do you think this might be an effective weight-loss drug?
Show Answer
After DNP poisoning, the electron transport chain can no longer form a proton gradient, and ATP synthase can no longer make ATP. DNP is an effective diet drug because it uncouples ATP synthesis; in other words, after taking it, a person obtains less energy out of the food he or she eats. Interestingly, one of the worst side effects of this drug is hyperthermia, or overheating of the body. Since ATP cannot be formed, the energy from electron transport is lost as heat.
Chemiosmosis (Figure 9) is used to generate 90 percent of the ATP made during aerobic glucose catabolism; it is also the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation. Recall that the production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation. The overall result of these reactions is the production of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the end of the pathway, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen attract hydrogen ions (protons) from the surrounding medium, and water is formed.
Practice Question
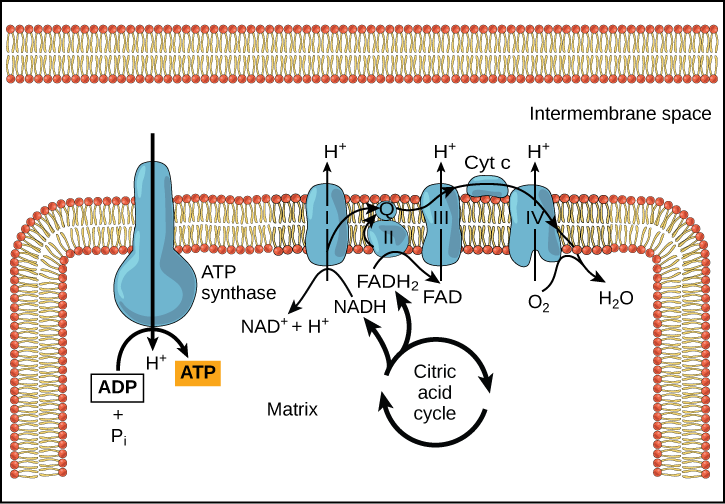
Figure 9. In oxidative phosphorylation, the pH gradient formed by the electron transport chain is used by ATP synthase to form ATP.
Cyanide inhibits cytochrome c oxidase, a component of the electron transport chain. If cyanide poisoning occurs, would you expect the pH of the intermembrane space to increase or decrease? What effect would cyanide have on ATP synthesis?
Show Answer
After cyanide poisoning, the electron transport chain can no longer pump electrons into the intermembrane space. The pH of the intermembrane space would increase, the pH gradient would decrease, and ATP synthesis would stop.
ATP Yield
The number of ATP molecules generated from the catabolism of glucose varies. For example, the number of hydrogen ions that the electron transport chain complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons across the membranes of the mitochondria. (The NADH generated from glycolysis cannot easily enter mitochondria.) Thus, electrons are picked up on the inside of mitochondria by either NAD+ or FAD+. As you have learned earlier, these FAD+ molecules can transport fewer ions; consequently, fewer ATP molecules are generated when FAD+ acts as a carrier. NAD+ is used as the electron transporter in the liver and FAD+ acts in the brain.
Another factor that affects the yield of ATP molecules generated from glucose is the fact that intermediate compounds in these pathways are used for other purposes. Glucose catabolism connects with the pathways that build or break down all other biochemical compounds in cells, and the result is somewhat messier than the ideal situations described thus far. For example, sugars other than glucose are fed into the glycolytic pathway for energy extraction. Moreover, the five-carbon sugars that form nucleic acids are made from intermediates in glycolysis. Certain nonessential amino acids can be made from intermediates of both glycolysis and the citric acid cycle. Lipids, such as cholesterol and triglycerides, are also made from intermediates in these pathways, and both amino acids and triglycerides are broken down for energy through these pathways. Overall, in living systems, these pathways of glucose catabolism extract about 34 percent of the energy contained in glucose.
In Summary: Electron Transport Chain
The electron transport chain is the portion of aerobic respiration that uses free oxygen as the final electron acceptor of the electrons removed from the intermediate compounds in glucose catabolism. The electron transport chain is composed of four large, multiprotein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers shuttling electrons between them. The electrons are passed through a series of redox reactions, with a small amount of free energy used at three points to transport hydrogen ions across a membrane. This process contributes to the gradient used in chemiosmosis. The electrons passing through the electron transport chain gradually lose energy, High-energy electrons donated to the chain by either NADH or FADH2 complete the chain, as low-energy electrons reduce oxygen molecules and form water. The level of free energy of the electrons drops from about 60 kcal/mol in NADH or 45 kcal/mol in FADH2 to about 0 kcal/mol in water. The end products of the electron transport chain are water and ATP. A number of intermediate compounds of the citric acid cycle can be diverted into the anabolism of other biochemical molecules, such as nonessential amino acids, sugars, and lipids. These same molecules can serve as energy sources for the glucose pathways.
Let's Review
Cellular respiration is a collection of three unique metabolic pathways: glycolysis, the citric acid cycle, and the electron transport chain. Glycolysis is an anaerobic process, while the other two pathways are aerobic. In order to move from glycolysis to the citric acid cycle, pyruvate molecules (the output of glycolysis) must be oxidized in a process called pyruvate oxidation.
Glycolysis
Glycolysis is the first pathway in cellular respiration. This pathway is anaerobic and takes place in the cytoplasm of the cell. This pathway breaks down 1 glucose molecule and produces 2 pyruvate molecules. There are two halves of glycolysis, with five steps in each half. The first half is known as the "energy requiring" steps. This half splits glucose, and uses up 2 ATP. If the concentration of pyruvate kinase is high enough, the second half of glycolysis can proceed. In the second half, the "energy releasing: steps, 4 molecules of ATP and 2 NADH are released. Glycolysis has a net gainof 2 ATP molecules and 2 NADH.
Some cells (e.g., mature mammalian red blood cells) cannot undergo aerobic respiration, so glycolysis is their only source of ATP. However, most cells undergo pyruvate oxidation and continue to the other pathways of cellular respiration.
Pyruvate Oxidation
In eukaryotes, pyruvate oxidation takes place in the mitochondria. Pyruvate oxidation can only happen if oxygen is available. In this process, the pyruvate created by glycolysis is oxidized. In this oxidation process, a carboxyl group is removed from pyruvate, creating acetyl groups, which compound with coenzyme A (CoA) to form acetyl CoA. This process also releases CO2.
Citric Acid Cycle
The citric acid cycle (also known as the Krebs cycle) is the second pathway in cellular respiration, and it also takes place in the mitochondria. The rate of the cycle is controlled by ATP concentration. When there is more ATP available, the rate slows down; when there is less ATP the rate increases. This pathway is a closed loop: the final step produces the compound needed for the first step.
The citric acid cycle is considered an aerobic pathway because the NADH and FADH2 it produces act as temporary electron storage compounds, transferring their electrons to the next pathway (electron transport chain), which uses atmospheric oxygen. Each turn of the citric acid cycle provides a net gain of CO2, 1 GTP or ATP, and 3 NADH and 1 FADH2.
Electron Transport Chain
Most ATP from glucose is generated in the electron transport chain. It is the only part of cellular respiration that directly consumes oxygen; however, in some prokaryotes, this is an anaerobic pathway. In eukaryotes, this pathway takes place in the inner mitochondrial membrane. In prokaryotes it occurs in the plasma membrane.
The electron transport chain is made up of 4 proteins along the membrane and a proton pump. A cofactor shuttles electrons between proteins I–III. If NAD is depleted, skip I: FADH2 starts on II. In chemiosmosis, a proton pump takes hydrogens from inside mitochondria to the outside; this spins the "motor" and the phosphate groups attach to that. The movement changes from ADP to ATP, creating 90% of ATP obtained from aerobic glucose catabolism.
Let's Practice
Now that you've reviewed cellular respiration, this practice activity will help you see how well you know cellular respiration:
Click here for a text-only version of the activity.
Check Your Understanding
Answer the question(s) below to see how well you understand the topics covered in the previous section. This short quiz doesnot count toward your grade in the class, and you can retake it an unlimited number of times.
Use this quiz to check your understanding and decide whether to (1) study the previous section further or (2) move on to the next section.
Source: https://courses.lumenlearning.com/suny-wmopen-biology1/chapter/cellular-respiration/
0 Response to "Active Cells Must Continually Produce Atp"
Post a Comment